Information management systems are essential to health systems playing a number of critical functions including supporting efficient registration and management of medical products for timely access and availability of medicines, vaccines and diagnostics.
Through this project, open source software will be made available for installation that will facilitate medical product registration and stock management of medical products across health system levels. A cold chain monitoring module will also be developed with automated temperature monitoring, alerts and visualisation networked throughout the supply chain, which will safeguard the appropriate storage of medical products including vaccines.
- For product registration an existing regulatory information management software (Integrated Regulatory Information Management System, IRIMS), will be redeveloped as open source with new functionality integrated into the mSupply support system (Open IRIMS). The new software will be deployed in Laos.
- mSupply desktop is a licenced medical inventory software that is widely used in low and middle income countries to support procurement, distribution and dispensing of medical stocks. The software will be redeveloped to first phase as open source (Open mSupply).
- Alongside this, a cold chain monitoring module will be expanded and integrated into mSupply to allow close management of cold chain supplies from fridges in central warehouses through to primary health facilities.
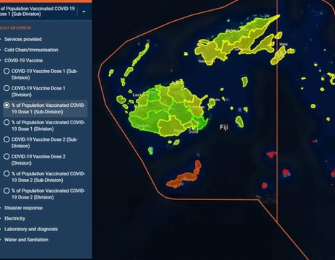
The integration of a cold chain monitoring module into mSupply supports tracking of individual COVID-19 vaccine batches complete cold chain storage history from procurement through to patient dispensing. Thus ensuring the quality and safety of vaccines during immunisation rollout.
Impact So Far:
This investment has:
- With support from the TGA, deployed an existing regulatory information management software, Integrated Regulatory Information Management System (IRIMS) in Laos.
- Redeveloped IRIMS as open source, flexible regulatory information management system, called Conforma.
- Conforma is planned to be rolled out in Fiji.
- Conforma is able to manage various regulatory tasks that include Pharmaceutical Company Licensing, New Drug Registrations and Medicine Import / Export Permits.
- Supported the development of an open source version of the popular mSupply, which is a licenced pharmaceutical inventory software that enables the procurement, distribution and dispensing of medical stocks. The newly developed Open mSupply has been rebuilt from the ground up and is now available.
- Added a cold chain monitoring module into mSupply to allow close management of cold chain supplies from fridges in central warehouses through to primary health facilities. This functionality has supported the cold chain storage history and tracking from procurement through to patient dispensing of COVID-19 vaccines. Thus ensuring the quality and safety of vaccines.